The below post is something I am sharing from an assignment I wrote recently. It is long, though I hope it is of use to some of you. All sources are at the end, and I strongly suggest the books I have referenced you buy – they’re all absolutely fantastic books. Particular kudos goes to Dujesiefken & Liese’s ‘The CODIT Principle’, which is hands-down brilliant.
The compartmentalisation process can be split into four sections, under the banner of two governing parts. The four stages are: (1) wounding, (2) response of the tree to compartmentalise the injury and possible infection, (3) the succession of micro-organisms that seek to actively or passively combat the tree’s natural defence mechanisms, and (4) development of subsequent wood discolouration and decay. The two governing parts are: (1) events that occur within the wood at the time of wounding, and (2) events that occur afterwards (Shigo, 1991). However, there are many aspects that we likely yet do not properly understand, or even understand at all.
It is also necessary to recognise that, by default, trees are hugely compartmented (Karban, 2015; Shigo, 1986). Wood is split up by growth rings and radial rays into small sections, single cells are internally compartmented into many different functional units and are themselves segmented into groups by radial and terminal parenchyma, and branches are not conductively attached to the trunk tissue from above. Certain species will also form heartwood, which further compartmentalises the tree. Compartmentalisation is nothing new to a tree, therefore. There is nonetheless a difference – natural growth form leads to high levels of ‘compartmentation’, whilst ‘compartmentalisation’ is a concept that relates to resisting decay exclusively (Shigo, 1986).
The overall concept of phenotypic response to infection is determined by not only genetics (Santamour, 1979; Santamour, 1984a; Santamour, 1984b; Shigo, 1986; Shigo, 1991), though also energy levels (Shigo, 1986; Shigo, 1991; Wargo, 1972; Wargo, 1976; Wargo, 1977). Where energy reserves are high, the compartmentalisation of decay will be more effective than if energy levels are low. When a tree that has poor energy reserves is infected, the pathogen will thus have an easier means of establishment internally, further reducing already-depleted reserves. Therefore, CODIT mechanisms, whilst dictated by genetic qualities primarily, are heavily influenced by the energy available to the tree. Without needed energy reserves, not even a mighty oak may be able to compartmentalise effectively.
It should also be noted that phenology plays a role in successful defence (Shigo, 1991). All trees have their weak points, and therefore at times the response to wounding will inherently be less favourable, given the traits of the tree. Such a weakness may be exacerbated if the period of acute weakness aligns with the period of maximum strength of the infecting pathogen.
In essence, compartmentalisation is a truly dynamic process. Reaction zones are constantly shifting in response to pathogenic activity (sometimes on multiple fronts from multiple wounds). This favours everything involved within the process, as a state of immobility in nature means death. Despite this, the tree always has the upper hand, as the pathogen that wins the battle to begin infecting its host may (1) not be the pathogen that is best-suited to attack the specific host tree, and / or (2) the pathogen does not know what it can expect in terms of resistance by the tree – the tree’s defence system responds to pathogen presence, in a sort of unexpected counter-attacking measure that the pathogen cannot (accurately) anticipate (Shigo, 1986).
With all this in mind, we can now explore the effectiveness of the physiological responses a tree will go through following wounding.
Physiological changes
As previously ascertained, the CODIT model is segmented into the reaction zone (walls I-III), and the barrier zone (wall IV). Whilst all four walls resist the spread of dysfunction, they are not all as effective as one another. In fact, the relative strength of each wall increases from I through to IV.
Wall I, which resists vertical spread of dysfunction from the wound site, is not a biological feature. It is nonetheless under strong genetic control. It provides the weakest of all the wall defences, but this makes the most sense when one recognises that a tree requires vascular connectivity and function for all of its life processes – if too many vascular elements are plugged, other problems may arise (Shigo et al., 1977). Additionally, any vertical spread of infection is largely without significant risk, as the new growth rings formed in the years following will ensure the tree retains an element of vascular functionality (Shigo, 1986). Where this wall is particularly weak, extensive vertical columns of discolouration and decay will likely manifest (Shigo, 1991).
Tyloses, which are balloon-like structures, form from the parenchyma cells that border the vascular tissues, and thus facilitate with the plugging of conductive vessels within angiosperms. The pits, which border the vessels, ensure vascular functionality is retained by promoting the localised lateral movement of water around the plugged vessels (Watson, 2006). This adaption ensures the tree can still operate with relative efficiency. Further, depending on the abundance of parenchyma cells around the vessels, the effectiveness of wall I may be very weak or rather robust. Quercus spp. do, for instance, have parenchyma cells that surround all large vessels in the earlywood, whereas other species may only see parenchyma cells surround a portion of the vessels – Ulmus spp. are but one genus with this characteristic, as their earlywood vessels are so massive and latewood vessels are so densely clustered together that parenchyma cells cannot envelop the entire vascular network. However, the vessel structure of elm means that wood is essentially more air than it is wood, which also acts as a defensive mechanism against pathogen invasion (Shigo, 1986).
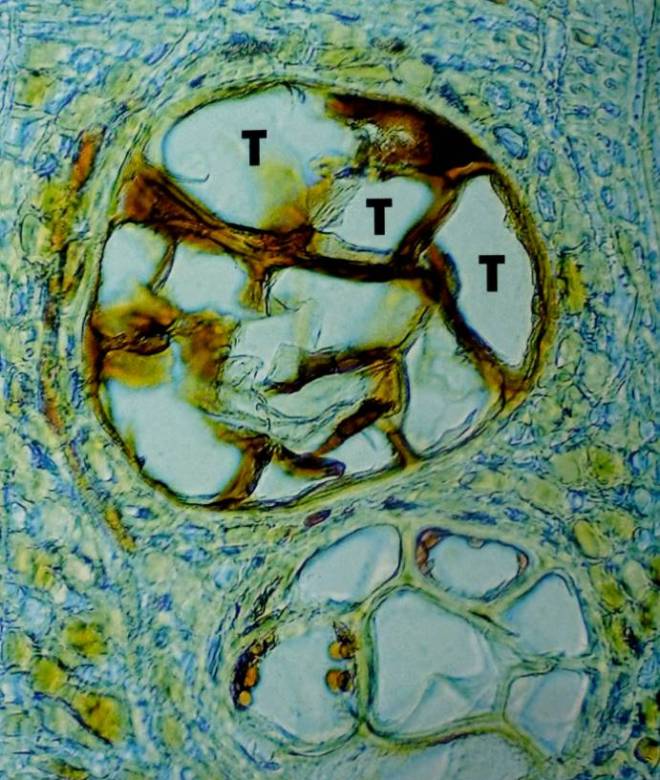
In particular species, the tyloses are also suberised – Prunus avium, Quercus rubra, and Ulmus americana are just three examples (Biggs, 1987). Such suberisation will aid with the ability of wall I to resist pathogenic spread in a vertical manner, though this does not cause the wall to rank above others in terms of efficacy.
The second wall, which resists inward spread of dysfunction, is also not a biological feature and is, too, under strong genetic control. This wall is stronger than the first wall, and is strengthened post-wounding by tyloses and suberin within parenchyma cells (Shigo et al., 1977). Wall II is able to be ‘defeated’ by pathogens relatively easily, however – particularly in diffuse-porous woods, where latewood cells are not as densely clustered. The propensity of the wall being breached, therefore, ultimately relates to the thickness of the terminal parenchyma band within the previous year’s latewood – Ulmus spp. will suffer, as their terminal parenchyma are very thinly spread (Shigo, 1986). Where this wall is breached, a deep decay column will form (Shigo, 1991).

Wall III, which constitutes the existing radial rays within the wood structure, much like wall II, is strengthened post-wounding. This wall provides a better likelihood of protection against decay than the first and second walls respectively, mainly because (at least in broadleaves; not so much on conifers) the radial parenchyma sheets are very thick (many single sheets of parenchyma cells very close together) (Shigo, 1986; Shigo et al., 1977; Thomas, 2000). The fact that no ray will come into contact with another ray is a further safeguarding measure (Dujesiefken & Liese, 2015), as this nullifies the risk of a fungal pathogen using rays as vectors for internal establishment. An increased abundance of radial parenchyma sheets will also impact beneficially upon efficacy of this wall. Where this wall fails, decay will spread inwards and laterally. Where it is successful, decay will be ‘channelled’ between the two successful rays. Depending on the success of wall II however, the decay may still propagate deep into the wood structure (Shigo, 1986; Shigo, 1991).
The barrier zone, or wall IV, differs from the reaction zone walls I-III, in the sense that the resultant structure is anatomically different, and separates infected wood from the healthy wood laid down following wounding. Formed from living parenchyma tissues within the cambium that existed at the time of wounding, the barrier zone is initially heavily lignified, though over time suberin accumulates within the new cells’ walls and provides these cells with an impermeable property, preventing propagation of the pathogen into the ‘new wood’ (Biggs, 1985; Biggs, 1986; Shigo, 1986). As already elucidated to, the rate of suberin accumulation and its overall abundance will impact upon success of the wall. The form that the barrier zone adopts is influenced by wound severity, the time of wounding, the species of invading pathogen(s), and the host-specific traits. Some species will form very strong barrier zones and weak reaction zones, whilst other species may be inversely-specialised (Shigo, 1991).
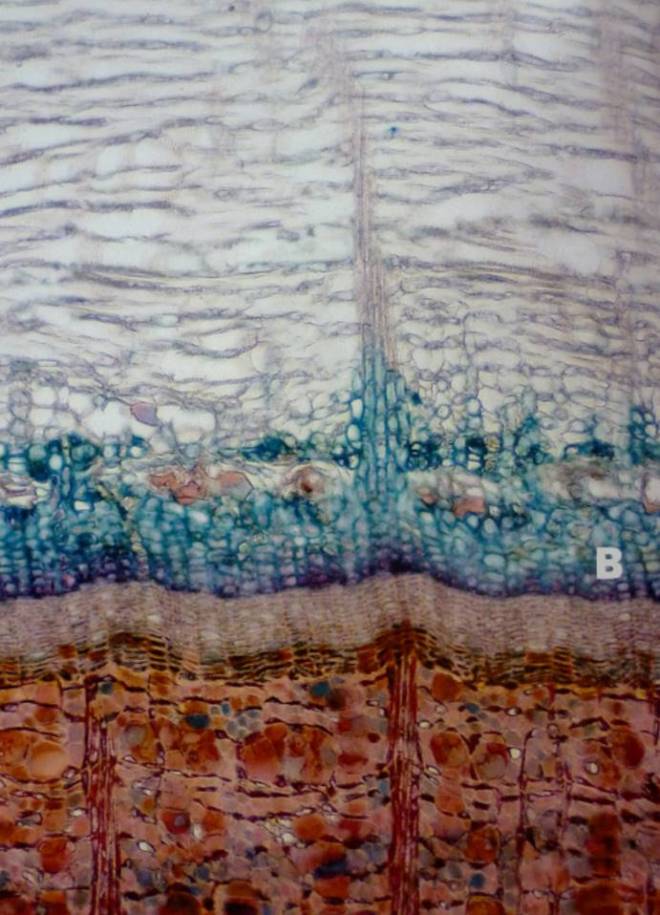
Decay rarely spans out into the new vascular tissues laid down following wounding, as the barrier zone is comprised of short parenchyma cells. Further, as the new wood of the barrier zone is hydraulically and structurally ‘sound’, it is free of dysfunction and therefore highly resistant to decaying organisms (Lonsdale, 1999; Shigo, 1991); that is, unless, another wound occurs in the new barrier zone tissues, at which point the process begins again. At times, this new wound can be self-inflicted, as the barrier zone is prone to splitting under loading conditions. This is caused by the propensity for the rolling woundwood ribs to not properly adjoin – the rolling ribs may, at times, turn inward, creating radial ‘shakes’ that act as internal cracks (Shigo, 1986). An incompletely closed wound may also provide an excellent environment for fungal pathogens, and it is possible that pathogenic micro-organisms influence the direction of growth of new vascular cambium (Blanchette & Biggs, 1992). The barrier zone also doesn’t actually aid with structural stabilisation very well – its role is to retain the tree’s functional integrity, and not so much its structural integrity (Shigo et al., 1977). Alternatively, the barrier zone can be broken by insect borers. In fact, insects are rather common in trees where wounding has occurred, as the decaying wood within is an ideal substrate for larvae. Such tunnelling by the insect therefore penetrates through barriers, and the tree cannot do anything but look to further wall-off itself (Shigo, 1986), which makes it progressively harder to repeat the same processes as there is less energy available for storage and thus for use.
Interestingly, despite the barrier zone’s resistance to the spread of dysfunction into new wood, certain species of fungi, such as Inonotus hispidus, can penetrate the barrier zone (Lonsdale, 1999; Thomas, 2000). Furthermore, the wounding site is most beneficial to fungal growth when woundwood has almost occluded the wound, though once closed, fungal infection of the site will usually stop (unless oxygen is supplied from elsewhere) (Shigo, 1986).
Ultimately however, even when walls I-III fail, wall IV is usually steadfast in its defence.
Further comments regarding physiological changes
The reaction zone (walls I-III) is, in the best cases, able to fully restrict fungal infection further into the wood after wounding, though the success of the reaction zone is largely related to carbohydrate availability in adjacent tissues, in addition to ample water so to combat against the transpirational loss triggered by the death of the exposed wound area’s xylem tissues. Such water stress in particular can cause wood to dry out further away from the wounding site, therefore improving the conditions for successful fungal establishment (Lonsdale, 1999). One can therefore stipulate that the health of the tree prior to wounding plays a significant role in the ability for the tree to resist the fungal attack. It should be noted here that seasonal changes in carbohydrate reserves, and the accompanying changes in wood moisture content, have been shown to alter successful invasion rates of fungi such as Chondrostereum purpureum (Schwarze & Baum, 2000).
Furthermore, the reaction zone is not structurally homogeneous and does not therefore form a continuously-similar morphological barrier to the ingress of air and / or fungal attack (Schwarze & Baum, 2000). The research by Schwarze & Baum (2000) also demonstrates that, where reaction zones should migrate alongside the internal progression of fungal pathogens (or other dysfunction), various fungal species (such as Ganoderma adspersum, Inonotus hispidus, and Kretzschmaria deusta) are able to extend into functional sapwood some way beyond the reaction zone, whilst not stimulating the simultaneous migration of the reaction zone. Instead, a new reaction zone forms later at the new colonization front and, much like its predecessor, there is no guarantee this reaction zone won’t befall the same fate.
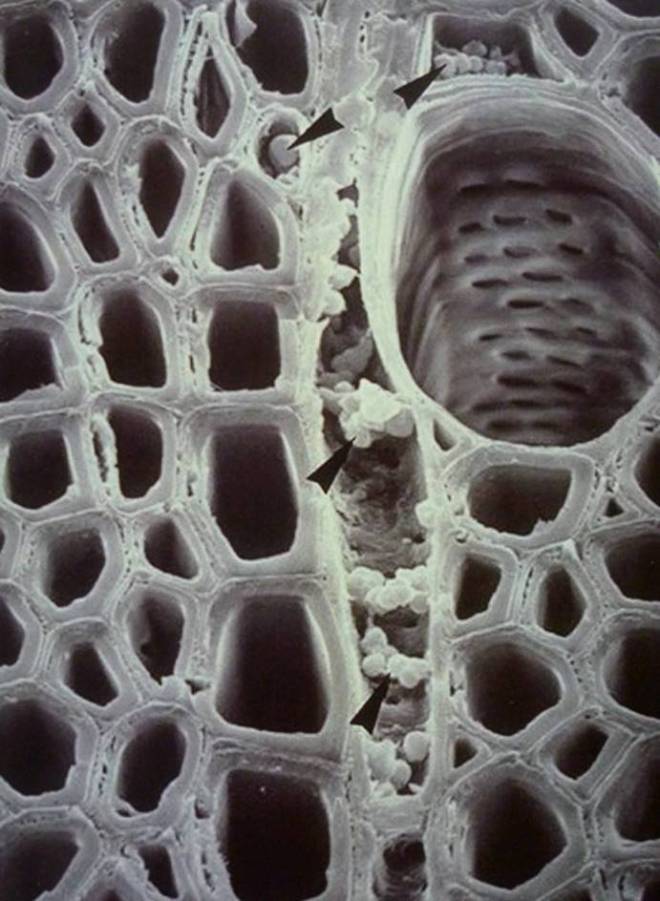
However, one must recognise that trees may have hundreds – if not thousands – of decayed and discoloured patches amongst their structure. By-and-large, the compartmentalisation process is successful, and most injuries are small (from small insect bore holes, dead twigs, etc), though it only takes one instance of failure (usually with more serious wounds) for dysfunction of any kind to wreak havoc on the tree’s system. This risk also increases over time, after each instance of compartmentalisation, as the locked-away resources and storage parenchyma cells cannot be accessed or used any longer (Shigo, 1986).
Furthermore, the chemically-strengthened reaction zone around a wound is, in addition to being loaded with suberin and parenchyma cells, laden with phenolic compounds that act as a toxin to most pathogens present. These phenols are toxic not only to invading micro-organisms, but also to the tree itself (Shigo et al., 1977). Despite this, substantial degradation of phenolic compounds by Ganoderma adspersum and Ganoderma resinaceum has been observed in a range of broadleaved species (Schwarze & Baum, 2000). Therefore, phenols are not necessarily an absolutely sure-fire combatant against fungal pathogenicity. In essence however, the tree segments part of its structure to act as the sacrificial lamb. Of course, the tree most usually survives because, alongside the fortification provided by the reaction zone it is also generating new, healthy wood from the cambium, though it should be noted that a tree cannot fully compartmentalise all of its structure as in doing so it will die (Shigo, 1991) – there comes a point where compartmentalisation simply cannot occur any further, as the loss in accessible parenchyma cells for energy storage reaches a critical point where life processes cannot be sustained.
Diverting attention again towards the barrier zone, it is necessary to recognise that the production of callus tissue, the subsequent cellular differentiation within the vascular cambium of the callus, and the eventual closure of the wound (which usually results in fully functional vascular connectivity), occurs following wounds inflicted only up to the depth of the xylem – heartwood, or inactive wood, void of parenchyma cells that can morphologically differentiate into necessary tissues, is unable to respond to wounding in the same manner that sapwood can (Neely, 1988b; Shigo, 1986). The amount and rate of callus production following wounding will of course vary between and within species, the size of the wound, the location of the wound on the tree, and the local environmental conditions (light availability, rainfall patterns, and so on). For example, the higher on the stem an injury is located, the faster the wound is closed. Further, young and small trees overgrow wounds more effectively when compared to older or thicker trees (Neely, 1988a; Schneuwly-Bollschweiler & Schneuwly, 2012).
After significant wounding, trees might even produce additional sapwood and also stall heartwood formation, in order to bolster parenchyma cell abundance within the wounded region (or even throughout the entire circumference) (Shigo, 1991). Additionally, radial growth increases following wounding have been observed where drill bits were drilled nearly all of the way through the tree, though did not touch the cambium on the opposite side to the entry point. Curiously, the results of the drill tests indicate that the cambium tissue reacts even when not touched (or directly associated with the problem), and receives signals via the symplastic components of the overall structure (Shigo, 1986) – this crucial vascular connectivity further adds context to the reason why wall I is the weakest of the four walls.
Moving away from the four walls on an exclusive basis and broadening the horizons of resistance to dysfunction as a whole, there are many other observable physiological mechanisms that a tree goes through following wounding. One significant aspect of resisting pathogens, whilst previously described in brief, is suberin accumulation. Suberin accumulation is an important process within the cell walls of new tissues that have formed after wounding, but before the internal spread of the pathogen is resisted to any marked degree. It does also occur within the lamella on the inner wall of a cell, which is already present at the time of wounding, though this lamella differentiates into a ligno-suberized layer following any such wounding (Biggs & Miles, 1988). The rate at which an infected site can accumulate suberin is directly related to the resistance to pathogen spread, as established. Therefore, trees that can flush the wound site with suberin stand a better chance of resisting infection (Biggs & Miles, 1985). However, suberin accumulation typically works in conjunction with, in angiosperms, the tyloses’ ‘plugging’ of conductive vessels. Suberin, on an exclusive basis, is not normally able to form homogeneous resistance (Biggs, 1987).
Furthermore, suberin and its relation to the barrier zone (it is present as a lining of the cells present within the barrier zone) is very much evident, and its abundance will therefore have direct relevance to the success of the barrier zone (Shigo et al., 1977; Shigo, 1986). Suberin is ultimately what provides the fourth wall with its microbial resistance; many – but not all – fungal species cannot break down suberin (Biggs, 1987). Additionally, the accumulation of suberin (and lignin) within the boundary zone is reliant upon temperature, with 0°C being the threshold (Biggs, 1986). For wounds to completely lignify and suberise therefore, it may take many hundreds of ‘degree days’, and thus the rate of ligno-suberisation absolutely impacts upon the resistance of the barrier zone to infection. Wounds will also decrease in infection potential over time as they lignify and suberise (Watson, 2006), with a more rapid process, which is related to preferred temperatures (that will vary between species), aiding with a more swift decline in infection potential (Biggs, 1986).
It is critical to note, however, that suberin is only produced by living cells (Deflorio et al., 2008), and therefore if a wound extends into the heartwood then suberin cannot play a role in particular areas of the wound (those spanning outside of the sapwood region). However, heartwood can ‘compartmentalise’. Where it is wounded, a boundary is formed of enzymes that react with oxygen or in response to the presence of chemical secretions by micro-organisms (Shigo, 1986; Shigo, 1991). Such chemical alteration of the heartwood ensures that fungal pathogens do not have free-reign of the heartwood. Interestingly, heartwood that has very high concentrations of extractives is generally found to be within the longest-lived trees (Shigo, 1986) – heartwood therefore is critical in the overall compartmentalisation process, without question. Pathogens will eventually break down the extractive-rich heartwood, however. The process is simply much slower. At this point, it is worth noting that tree species with false heartwood (including Acer spp., Fagus spp., Fraxinus spp.) do not form their ‘heartwood’ (that is simply a discoloured central column) regularly, but instead form such discoloured wood following branch death. The discoloured wood serves the same purpose as heartwood does, by-and-large. Discolouration in false heartwood species is initially a light pink to orange, with the wood only darkening upon infection (Shigo, 1986).
In some species, such as Acer spp. and Betula spp., injuries made in very late winter or early spring may ooze or drip sap for some days to weeks afterwards (Shigo, 1986). This is a normal response and aids with creating undesirable (anaerobic) conditions at the wounding site for micro-organisms. Conifers do the same with traumatic resin ducts (canals). As the sap clogs the vessels, oxygen availability progressively decreases. Because wood-decay fungi are aerobes, they cannot function where the sap has clogged the region. Further, the pressure flow is outward, not inward (Dujesiefken & Liese, 2015; Shigo, 1986; Shigo, 1991).
References
Biggs, A. (1985) Suberized boundary zones and the chronology of wound response in tree bark. Phytopathology. 75 (11). p1191-1195.
Biggs, A. (1986) Prediction of lignin and suberin deposition in boundary zone tissue of wounded tree bark using accumulated degree days. Journal of the American Society for Horticultural Science. 111 (5). p757-760.
Biggs, A. (1987) Occurrence and location of suberin in wound reaction zones in xylem of 17 tree species. Phytopathology. 77 (5). p718-725.
Biggs, A. & Miles, N. (1985) Suberin deposition as a measure of wound response in peach bark. HortScience. 20 (5). p903-905.
Biggs, A. & Miles, N. (1988) Association of suberin formation in uninoculated wounds with susceptibility to Leucostoma cincta and L. persoonii in various peach cultivars. Phytopathology. 78 (8). p1070-1074.
Blanchette, R. & Biggs, A. (1992) Defence mechanisms of woody plants against fungi. Hong Kong: Springer.
Deflorio, G., Johnson, C., Fink, S., & Schwarze, F.. (2008) Decay development in living sapwood of coniferous and deciduous trees inoculated with six wood decay fungi. Forest Ecology and Management. 255 (7). p2373-2383.
Dujesiefken, D. & Liese, W. (2015) The CODIT Principle: Implications for Best Practices. USA: International Society of Arboriculture.
Karban, R. (2015) Plant Sensing & Communication. USA: University of Chicago Press.
Lonsdale, D. (1999) Principles of Tree Hazard Assessment and Management (Research for Amenity Trees 7). London: HMSO.
Neely, D. (1988a) Tree wound closure. Journal of Arboriculture. 14 (6). p148-152.
Neely, D. (1988b) Wound closure rates on trees. Journal of Arboriculture. 14 (10). p250-254.
Santamour, F. (1979) Inheritance of wound compartmentalization in soft maples. Journal of Arboriculture. 5 (10). p220-225.
Santamour, F. (1984a) Early selection for wound compartmentalization potential in woody plants. Journal of Environmental Horticulture. 2 (4). p126-128.
Santamour, F. (1984b) Wound Compartmentalization in Cultivars of Acer, Gleditsia, and Other Genera. Journal of Environmental Horticulture. 2 (4). p123-125.
Schneuwly-Bollschweiler, M. & Schneuwly, D. (2012) How fast do European conifers overgrow wounds inflicted by rockfall?. Tree Physiology. 32 (8). p968-975.
Schwarze, F. & Baum, S. (2000) Mechanisms of reaction zone penetration by decay fungi in wood of beech (Fagus sylvatica). New Phytologist. 146 (1). p129-140.
Shigo, A. (1986) A New Tree Biology. USA: Shigo and Trees Associates.
Shigo, A. (1991) Modern Arboriculture. USA: Shigo and Trees Associates.
Shigo, A., Marx, H., & Carroll, D. (1977) Compartmentalization of decay in trees. USDA Forest Service Agricultural Information Bulletin 405.
Thomas, P. (2000) Trees: Their Natural History. UK: Cambridge University Press.
Wargo, P. (1972) Defoliation-induced chemical changes in sugar maple roots stimulate growth of Armillaria mellea. Phytopathology. 62 (11). p1278-1283.
Wargo, P. (1976) Variation of starch content among and within roots of red and white oak trees. Forest Science. 22 (4). p468-471.
Wargo, P. (1977) Wound closure in sugar maple: adverse effects of defoliation. Canadian Journal of Forest Research. 7 (2). p410-414.
Watson, B. (2006) Trees – Their Use, Management, Cultivation, and Biology. India: The Crowood Press.
To discuss this post, please leave either a post below or over on Arbtalk.
































